Background: Mixed‐phenotype acute leukemias (MPAL), which is included in acute leukemias of ambiguous lineage (ALAL), can be classified as four different subtypes based on recurring genetic alterations including BCR::ABL, KMT2A and ZNF384 gene rearrangement as well as BCL11B activation. The 2022 WHO classification has already mentioned PICALM::MLLT10 fusions are also enriched in MPAL but need more data. In 2022 ELN classification, PICALM::MLLT10 was identified as acute myeloid leukemia (AML) with other rare recurring translocations but not ALAL. In addition, there is no separate classification for PICALM::MLLT10 in the new ICC classification. Therefore, it is still confused whether PICALM::MLLT10 corresponds to AML or acute lymphoblastic leukemia (ALL) or ALAL.
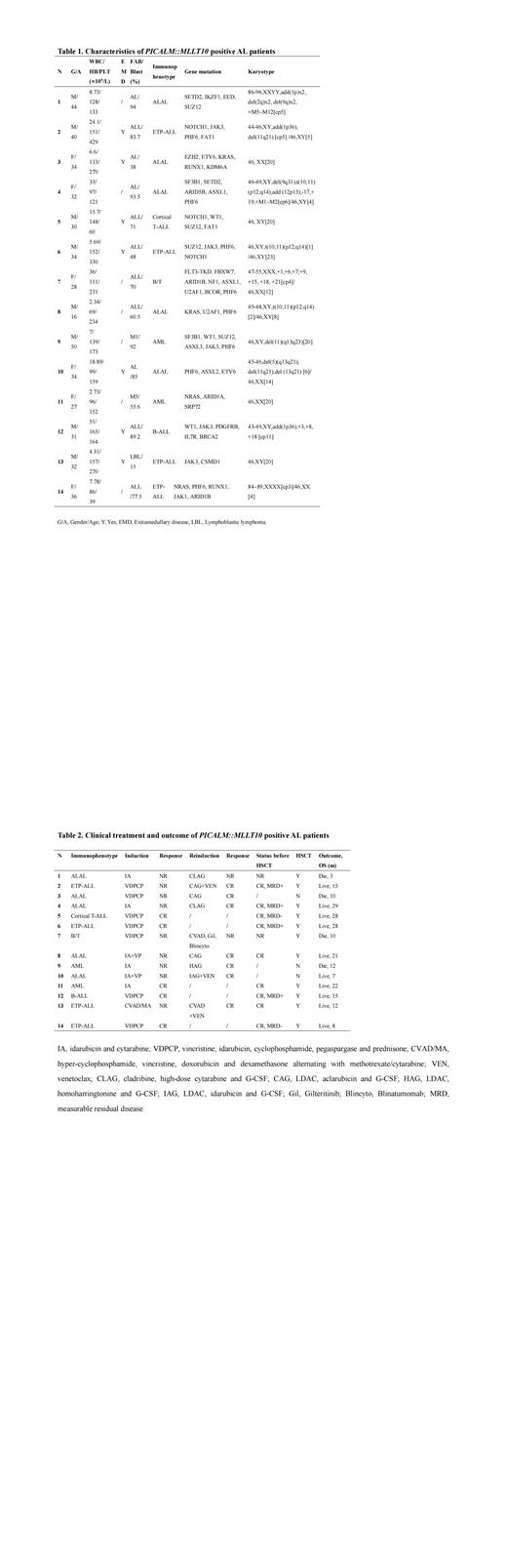
Methods: Fourteen PICALM::MLLT10 positive patients of 390 AL patients (14/390, 3.6%) were identified by RNA sequencing (RNA-seq) in our center from 2020-2022, including 8 female and 6 male, with a median age of 33 years (16-50 years). These patients were newly diagnosed AL according to bone marrow morphology and immunology including 5 ALAL, 5 T-ALL [ 4 early T-cell precursor ALL (ETP-ALL), 1 cortical T-ALL], 2 AML, 1 B-ALL with aberrant expression of myeloid antigen, and 1 B/T MPAL.
Results: The mean white blood cell counts of these patients was 8.25×10 9/L (2.34-51×10 9/L) and platelet counts was 168.5×10 12/L (39-429×10 12/L) individually. It should be mentioned that extramedullary disease (EMD) was found in 7 cases (7/14), including mediastinum, tonsil, and skin. In terms of immunotyping, CD7 was identified in all patients (14/14) and CD33 in 71.4% (10/14) patients. The major concurrence mutations were PHF6 mutation (8/14), JAK3 mutation (5/14), and SUZ12 mutation (4/14). Characteristic cytogenetic abnormality t(10;11)(p12.3;q14.2) was only found in three cases (3/14) (Table 1).
The PICALM breakpoints are mainly concentrated in exon 17 (n=6) and exon 19 (n=8). Exon 4 (n=8), exon 6 (n=2), exon 9 (n=2) and exon 10 (n=2) are the most commonbreakpointsof MLLT10. To our knowledge, this is the first report about the breakpoints and fusion gene forms of PICALM::MLLT10.
For initial treatment, these patients individually received standard ALL induction chemotherapy (VDPCP, vincristine, idarubicin, pegaspargase, cyclophosphamide, prednisone) and AML induction chemotherapy (3+7 IA regimen including idarubicin, cytarabine). The initial complete remission (CR) rate was only 35.7% (5/14), and 9 patients showing no remission (NR). These NR patients subsequently received salvage chemotherapy. It is worth mentioning that 6 of them received combined chemotherapy regimen including low doses of cytarabine (LDAC), granulocyte colony-stimulating factor (G-CSF) and anthracyclines such as aclarubicin or idarubicin or homoharringtonine (CAG or IAG or HAG), and 2 of 6 patients also further received combination therapy with venetoclax (CAG or IAG +VEN). Finally, 5 of them dramatically achieved CR (5/6). In the other 3 patients, 1 patient who was failure to initial Hyper-CVAD A/B regimen also dramatically achieved CR after the more Hyper-CVAD A regimen combined with venetoclax, and 1 patient achieved CR while another still NR with both receiving CLAG (cladribine, cytarabine, G-CSF) regimen. Subsequently 11 patients (9 CR, 2 with refractory disease) received allogenic hematopoietic stem cell transplantation (allo-HSCT), and all the CR patients survived well after HSCT (follow up 3-24 months, median 15 months) without relapse, while 2 NR patients died soon after transplantation because of severe complications (Table 2).
Conclusions: Our data suggested PICALM::MLLT10 positive AL should be more appropriately recognized as an independent ALAL entity and may benefit from LDAC, G-CSF and anthracyclines combination chemotherapy as well as venetoclax. Sequential HSCT after chemotherapy combined with venetoclax may further improve long-term survival in AL patients with CR even MRD positive.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal